

Artificial Intelligence (AI) model to predict the occurrence and timing of Adverse Events, thus enabling preemptive clinical interventions.
Adverse Event Prediction and Planning–Build AI models using FDA data (and/or proprietary data) to predict if and when a specific adverse event (AE) will occur and determine what are the leading patient, drug. and/or environmental factors driving an AE.
Company A collaborates on a groundbreaking drug, known for its rare adverse event (AE). They have meticulously gathered retrospective data on patients who experienced this rare AE.
Myra EB developed a sophisticated model based on clinical, laboratory, and radiologic data, designed to predict the occurrence and timing of this rare but serious AE. This innovation equips clinicians with advanced foresight, identifying patients at risk and preparing them to proactively address potential AEs.
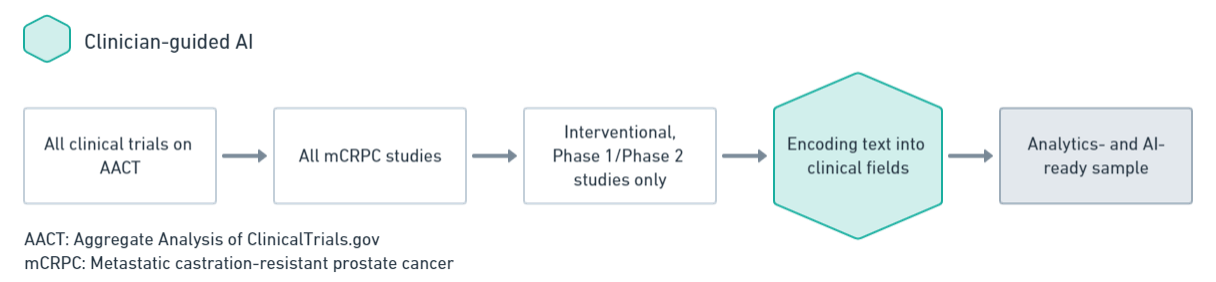
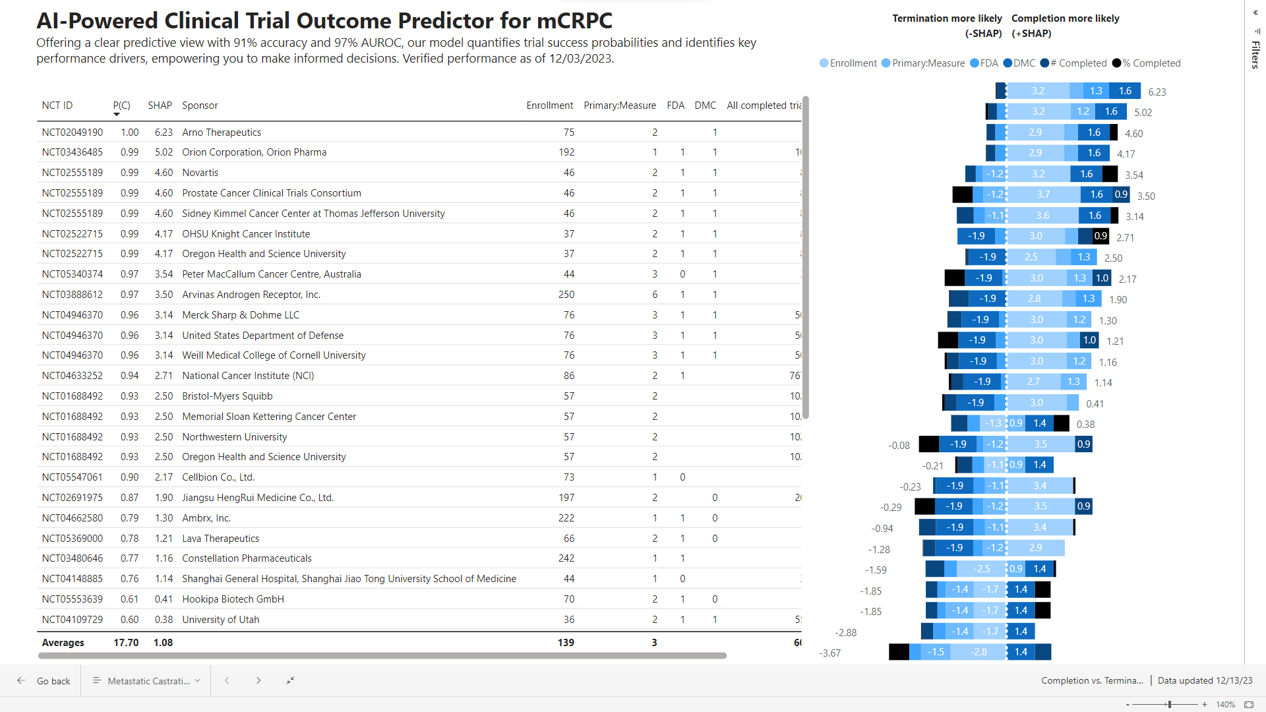
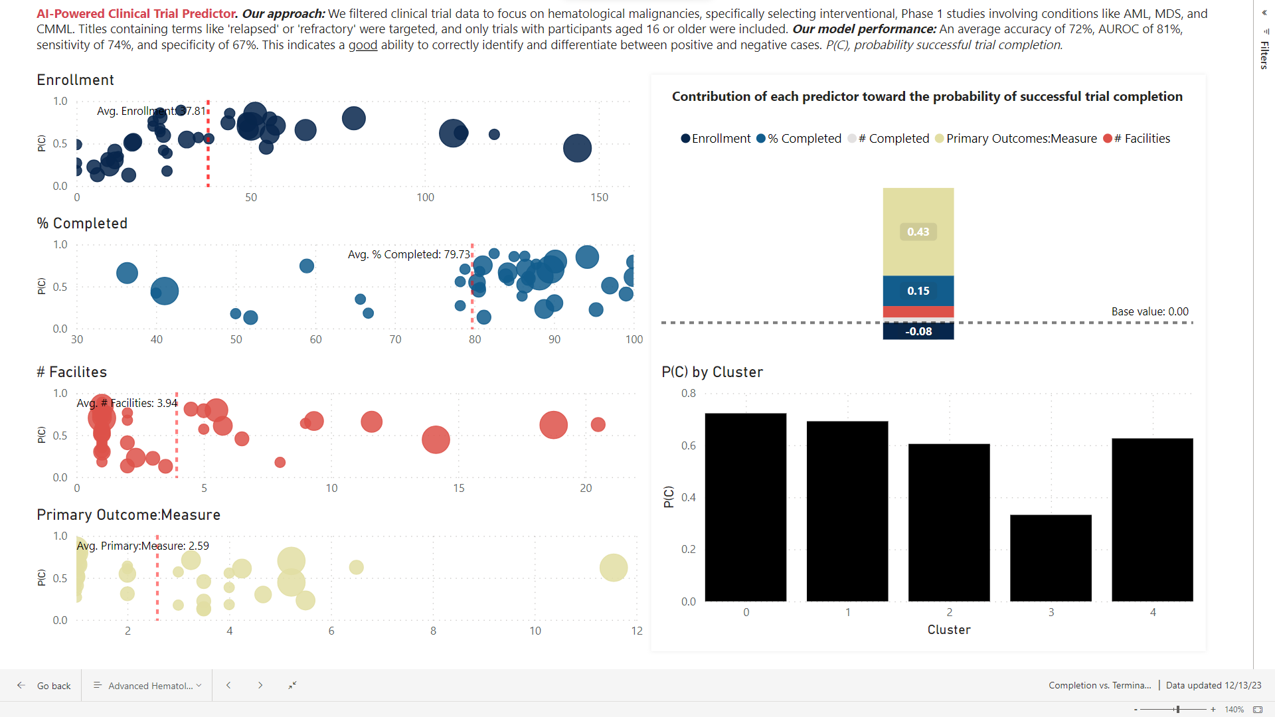
Precision Trial Design and Competitive Insights involve the development of AI models using historical and current trial data from data sources. These models are designed to predict the factors that drive trial completion versus termination, estimate the time to completion, and identify other crucial trial milestones.
Additionally, they enable the comparison of trials based on sponsor experience and the discovery of unique clusters of trial similarities. Furthermore, these insights help in determining which entities perform below and above industry standards, providing valuable competitive benchmarks.
“Our AI solution addresses key questions important to sponsors, such as ‘What actionable factors drive the success or failure of my trial? What strategies are others employing that lead to their success?
AI-Powered Interpretation & Integration: Advanced AI that interprets diverse radiological report styles for consistent data analysis with seamless integration of multimodal imaging reporting (MRI, CT, radiographs, etc.) for a unified analysis platform.
Advanced Data Processing & Standardization: Sophisticated algorithms for analyzing complex findings, particularly adept at cancer staging and intricate diagnoses, and conversion of variable medical text into a uniform JSON structure for downstream integration with analytical and clinical applications.
Intelligent Analysis & Clinical Decision Support: Predictive analytics employing pattern recognition across patient histories to identify health trends and inform prognoses, with an adaptive dashboard providing real-time data visualization to support clinical judgment and patient management.
Development & Compliance Framework: An extensible toolkit enabling custom solution development for varied clinical analytics needs. Comprehensive compliance and quality assurance protocols ensuring the integrity and regulatory adherence of data processing.
Continuous Learning & System Enhancement: A dynamic learning system that evolves, improving its algorithmic precision with each radiological report processed, ensuring ever-increasing accuracy and depth of data analysis.
Radiology-Enhanced Decision Support
Original Approach: Get a radiology report from a radiologist, have a trained technician or expert (not counting the amount of time spent training these people to know what to look for) read, interpret, extract data, and enter (correctly, no typos) into a database.
Our Approach: Get the radiology report, load into our secure application, load directly into the database. We use radiology reports, NOT IMAGES, to extract unstructured text data into structured datasets (e.g., JSON).
Impact: This saves a lot of time from manual data extraction, processing, and analysis. Our technology will reduce the report-to-data time significantly.
Remote Monitoring and Wearable Technology:
Remote monitoring and wearable technology enable the monitoring of patients participating in clinical trials from a distance. They facilitate real-time data collection, enhance patient engagement, and provide more comprehensive insights into the patients’ health.
Personalized Patient Engagement:
Artificial intelligence (AI) allows for the personalization of patient engagement strategies based on individual preferences, demographics, and health data. This may include tailored communication plans, reminders, and incentives to enhance patient involvement in clinical trials.
Patient Data:
AI analytics can process large datasets to derive insights into patient behavior, preferences, and experiences during a clinical trial. This information can be used to enhance future trials and improve overall patient engagement.
Adaptive Trial Design:
Adaptive trial designs have the capability to modify the study protocol based on accumulating data. This flexibility can lead to more efficient trials and better engagement by adjusting to emerging trends or patient responses.


Analyzing historical clinical trial data using machine learning can guide the selection of the most relevant clinical endpoints for a new trial.
For example, in the development of a heart disease drug, historical data might suggest that reducing the risk of heart attacks is a more meaningful endpoint than lowering cholesterol levels. Machine learning models can predict which endpoint is more likely to demonstrate the treatment’s efficacy, allowing pharmaceutical companies to focus their trial design on the most impactful endpoint.
This data-driven approach enhances the chances of regulatory approval and ensures that the trial aligns with the needs of patients and healthcare providers, ultimately optimizing the drug development process.
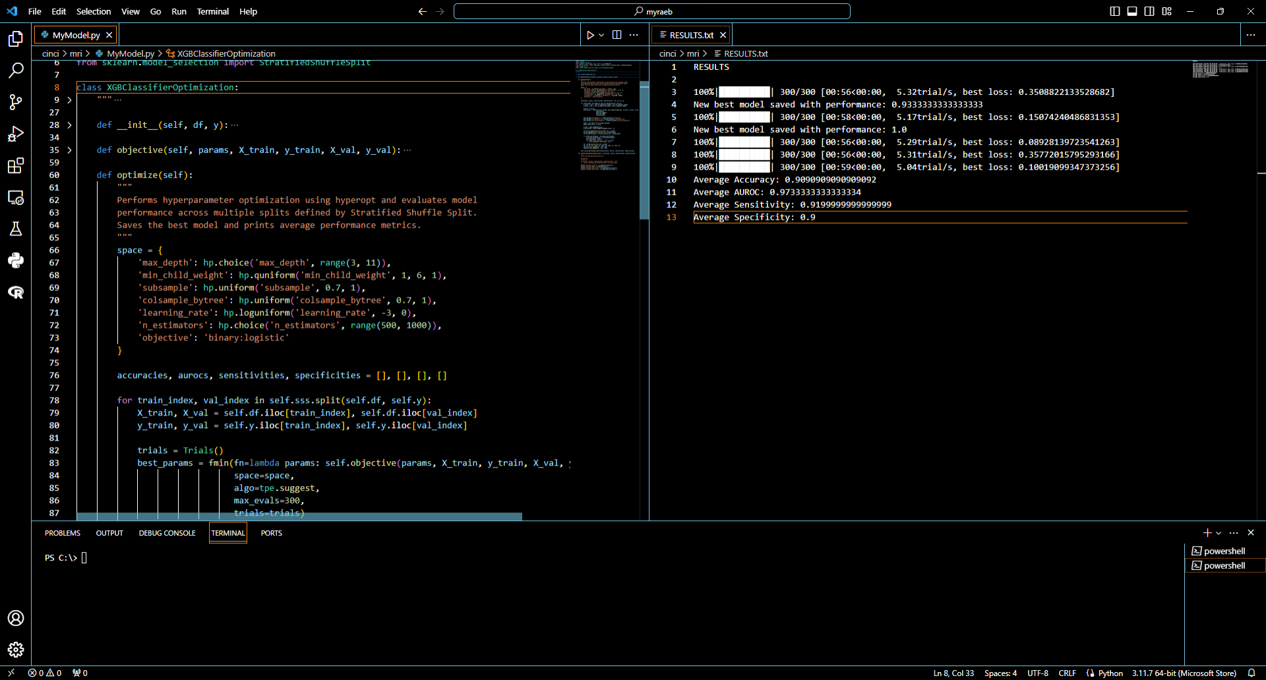
Utilizing advanced machine learning, our solution revolutionizes the clinical trial landscape by projecting precise trial timelines and optimizing participant recruitment strategies. We align seamlessly with clinical priorities, enabling meticulous enrollment criteria determination and resource allocation efficiencies. Furthermore, our AI models harness a wealth of historical trial data from both the NIH and ClinicalTrials.gov to predict trial costs with unprecedented accuracy. This empowers organizations to make informed financial decisions, optimize budgeting, and allocate resources judiciously, ultimately streamlining the entire trial process and enhancing the path to successful clinical outcomes.